Alzheimer’s disease (AD) is one of the most devastating neurodegenerative diseases. Aggregation of amyloid-beta peptide (Aβ) into cytotoxic oligomers and fibrills is one of the major hallmarks in AD. These pathological depositions in the brain are thought to be one of the main causes for the observed progressive cognitive decline in AD patients.
Interfering with Aβ aggregation is an inevitable strategy in the development of novel therapeutic approaches. Reliable in vitro models are needed, which are capable of showing direct effects of compounds on Aβ oligomer formation and thus, a beneficial impact on cell viability.
QPS Neuropharmacology provides fast and reproducible screening assays to investigate if your developmental compounds are able to protect against cytotoxic effects of aggregated Aβ1-42. Various in vitro models from primary neurons to several cell lines are available and described on the following pages.
Primary rat and mouse neurons
The hippocampus, a brain area critical for learning and memory, is especially vulnerable to damage at early stages of Alzheimer’s disease (AD). Primary rat/mouse hippocampal neurons are phenotypically closer to adult neurons as they have extended a dense network of neuronal processes.
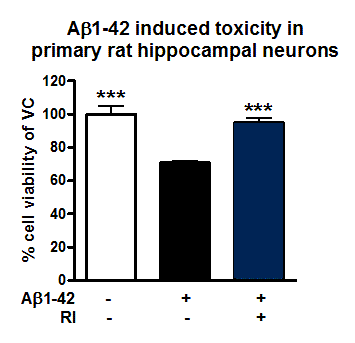
Figure: Neuroprotective effects of RI on Aβ1-42 induced toxicity in primary rat hippocampal neurons. Vehicle control, aggregated Aβ1-42 [1µM, 48h] or Reference item (RI) with aggregated Aβ1-42 were applied to primary rat hippocampal neurons. After 144h, cell viability was determined according to MTT assay. RI rescued cell viability and protected against toxic effects of Aβ1-42.
Primary chicken neurons
Primary chicken neurons secrete endogenous wildtype Aβ peptides. Both human and chick Aβ42 are identical in sequence.
Thus, primary chicken neurons are a suitable in vitro model for evaluating protective effects of your compounds on Aβ induced neurotoxicity.
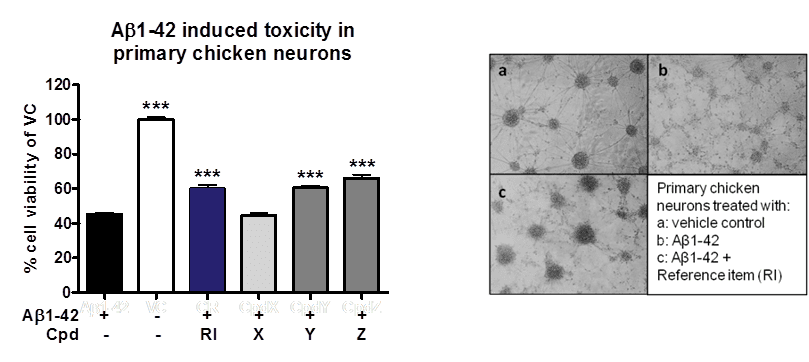
Figure: Neuroprotective effects of compounds on Aβ1-42 induced toxicity in primary chicken neurons. (A) Compounds were co-aggregated with Aβ1-42 for 48h and applied to primary chicken neurons on DIV6. After 96h, cell viability was determined according to MTT assay. Co-aggregation of compound Y and Z or reference item (RI) with Aβ1-42 rescued cell viability and protected against toxic effects of Aβ1-42. (B) Images illustrate neuroprotective effects of RI when co-aggregated with Aβ1-42 (c) in comparison to cells treated with aggregated Aβ1-42 only (b) or control cells (a).
SH-SY5Y cells
SH-SY5Y cells is a human derived neuroblastoma cell line and has become a popular in vitro model for neurodegenerative diseases.
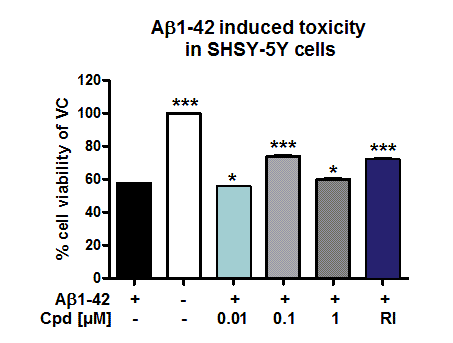
Figure: Evaluation of neuroprotective effects of compounds on Aβ induced toxicity in SH-SY5Y cells. Compounds were applied together with aggregated Aβ1-42 (1 µM) to SH-SY5Y cells for 96h. Cell viability was determined according to MTT assay. Reference item and Compound X (at 0.1 and 1µM) were able to protect against toxic effects of Aβ1-42.
More cell lines
Aβ1-42 toxicity assay is also available in:
- ARPE-19 cells (human Retina Pigment Epithelial cell line)
- PD cells (human fibroblasts from a PD patient)
- FRDA cells (human fibroblasts from a Friedreich Ataxia patient)
- LHON cells (human fibroblasts from a Leber‘s hereditary Optic Neuropathy)
Generation of Aβ species is a hallmark of Alzheimer’s Disease. QPS Neuropharmacology provides several cell culture models to identify pharmacological agents that interfere with the cleavage of APP by analyzing the generation of different Aβ and sAPP species by immunosorbent assay.
- Primary chicken telencephalic neurons secrete endogenous wildtype Aβ peptides, including Aβ38, Aβ40 and Aβ42. Both human and chick Aβ42 are identical in sequence. Therefore, this model is suitable for studying effects on processing of endogenous wildtype APP in primary neurons.
- Primary rat/mouse hippocampal neurons are phenotypically closer to adult neurons as they have extended a dense network of neuronal processes.
- H4 neuroglioma cells overexpressing human APPK595N/M596L, the Swedish double mutation, enable to analyze compounds for their effects on the human mutant APP profile.
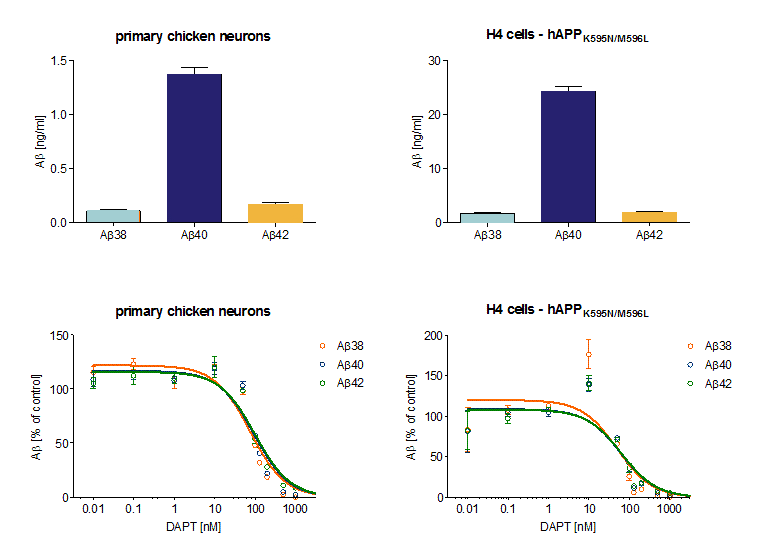
Figure: Aβ peptide profile in primary chicken neurons and APPK595N/M596L overexpressing cells.
(A) H4 cells overexpressing human APPK595N/M596L generated more Aβ38, 40, and 42 peptides in 24h than primary chicken neurons determined by an immunosorbent assay. In both, Aβ40 was the predominant Aβ peptide. (B) The γ-secretase inhibitor DAPT reduced Aβ formation in primary chicken neurons and in H4-hAPPK595N/M596L. In the H4 cell line, Aβ levels started to decrease at a concentration of 50nM in H4 cells and at 100 nM DAPT in chicken neurons after 24h treatment.
The pathological aggregation of amyloid-beta peptides (Aβ) is one of the major causes for the progressive cognitive decline in AD patients. The development of compounds interfering with Aβ aggregation and thus able to rescue neurodegeneration is indispensible. For this purpose, fast and reliable assays are needed capable of showing direct effects of compounds on Aβ oligomer formation.
QPS Neuropharmacology provides exclusively a fast and reproducible screening assay, Amorfix Aggregated Aβ Assay (A4), able to directly visualize beneficial effects of your developmental compounds on the formation of Aβ aggregates in vitro. Notably, compound Y and Z were able to reduce Aβ aggregate formation as determined by A4 assay.
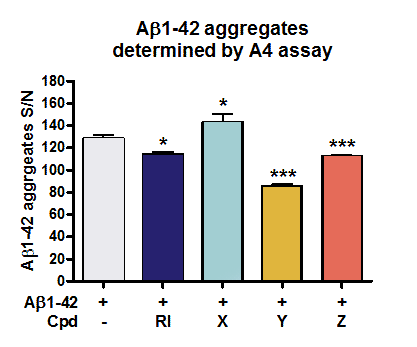
Figure: Determination of Aβ oligomers in vitro by A4 Assay. Compounds were co-aggregated with Aβ1-42 for 48h in vitro. Aggregated Aβ was separated from monomers through affinity interaction. After disaggregation, the originally aggregated Aβ was detected using a bead-based immunoassay that applies human Aβ-specific antibody labeled beads and Time Resolved Fluorescence (TRF) measurements. Aβ levels were evaluated as signal to noise ratio (S/N). Co-aggregation of compound Y and Z as well as reference item (RI) with Aβ1-42 reduced aggregate formation.
Tau proteins belong to the family of microtubule-associated proteins and play an important role in stabilizing the neuronal microtubules network. They are the major constituents of intraneuronal and glial fibrillar lesions described in Alzheimer’s disease and numerous neurodegenerative disorders referred to as ‘tauopathies’, including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Pick’s disease (PiD), argyrophilic grain disease, as well as the inherited frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17).
Molecular analysis revealed that hyperphosphorylation might be the important event leading to Tau aggregation resulting in neurodegeneration and dementia. Development of new compounds capable of preventing Tau hyperphosphorylation is an increasingly hot topic.
Thus, reliable models are needed that reflect Tau hyperphosphorylation in human diseases. For this purpose, we generated the stably transfected SH-SY5Y cell line over-expressing the longest human Tau441 isoform comprising two disease related mutations (V337M/R406W). The phosphorylation pattern of Tau in SH-SY5Y-TMHT441 cells can be reliably modulated by several kinase inhibitors. Effects on Tau phosphorylation (e.g. at Thr181, Ser202, Thr231/Ser235, Ser262 and Ser396/Ser4049) can be determined by various methods including immunosorbent assay (MSD), immunoblot analysis or mass spectrometry (Löffler T. et al, J Mol Neurosci 2012 May;47(1):192-203).
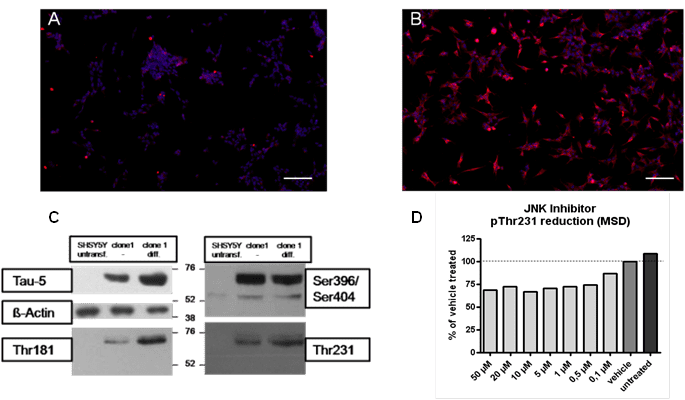
Figure: Tau hyperphosphorylation in SH-SY5Y-TMHT441 cells. Immunocytochemistry showing overexpression of human Tau in (A) untransfected and (B) transfected SH-SY5Y cells (Scale bar 100 µm). Human Tau is indicated in red, DAPI in blue. (C) Tau phosphorylation in control SH-SY5Y cells, in undifferentiated and differentiated SH-SY5Y-CMV-TMHT441 cells was examined by immuno blot analysis. (D) pThr231 was reduced in SH-SY5Y-CMV-TMHT441 cells after treatment with the JNK inhibitor SP600125 as assessed by an immunosorbent assay (MSD).
Hyperphosphorylation and accumulation of Tau in neurons is one of the main pathologic hallmarks in Alzheimer’s disease (AD) and other tauopathies, including Pick’s disease (PiD), argyrophilic grain disease, familial frontotemporal dementia and parkinsonism linked to chromosome 17.
Hyperphosphorylated Tau dissociates from microtubuli, resulting in the breakdown of the axonal flow, and thus impairs neuronal viability and function. Abnormal tau hyperphosphorylation is mainly induced due to the imbalance between protein kinases and phosphatases. To find promising drug candidates, inducible cellular models of Tau hyper-phosphorylation are useful screening tools for studying central nervous system drug effects.
Tau hyper-phosphorylation was induced by hypothermic conditions (30°C) in SH-SY5Y cells or SH cells overexpressing the longest isoform of human Tau 441 carrying two well-characterized mutations V337M/R406W (SH-SY5Y-Tau441). To reverse Tau hyperphosphorylation cells were treated with different kinase inhibitors like LiCl, a well known GSK3 beta inhibitor and were either kept under normo- or hypothermic conditions. Afterwards, total Tau and its phosphorylated species pSer262, pSer202, pSer396 and pThr231 were analyzed in cellular lysates by immunoblot or immunosorbent assay (Meso Scale Discovery, MSD).
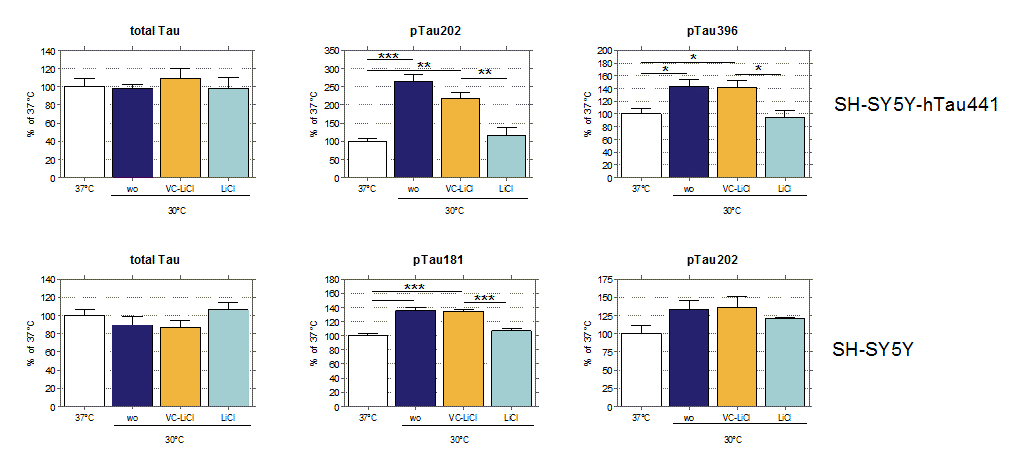
Figure: Hypothermia induced Tau hyper-phosphorylation is significantly reduced by LiCl in SH-SY5Y-Tau441 (top) and SH-SY5Y cells (bottom). Cells were subjected to 2h of hypothermia (30°C) and treated with either LiCl or Vehicle Control. Quantification of immunosorbent assay is shown of total Tau, pTau202, pTau181 and pTau396. Data are normalized to normothermic conditions. Data are shown as mean + SEM (n=4). Statistical significance is indicated by *<0.05, **<0.01, ***<0.001 as determined by One-Way ANOVA (Newman’s Keuls Multiple Comparison Test).
Tau protein stabilizes microtubules and contributes to key structural and regulatory cellular functions as axonal transport and signaling. Neurofibrillary tangles, mainly composed of bundles of Tau are implicated in the pathogenesis of neurodegenerative diseases such as tauopathies including Alzheimer‘s disease.
To identify inhibitors of Tau aggregation QPS provides two approaches:
The first approach is a spectrofluorometric read out that can be performed as Tau aggregation or dis-aggregation assay. The Tau aggregation assay aims to identify compounds that are able to mitigate the aggregation of Tau. The Tau dis-aggregation assay investigates if developmental compounds are able to reverse Tau aggregation.
These assays are cell-free in vitro assays using recombinant Tau441 (2N4R) P301L that is incubated in an aggregation buffer including ThioS. Fluorescence intensity is detected at 465 nm excitation and 510 nm emission.
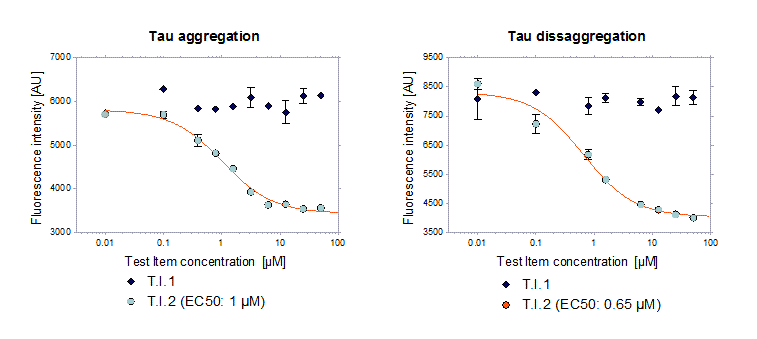
Figure 1: Test Item 2 minimized Tau aggregation in vitro and was also able to reduce pre-aggregated Tau. Test item 1 had no effect on Tau aggregation. EC50 was determined showing effective concentrations at 1 and 0.65 µM in the Tau aggregation and Tau dis-aggregation assay, respectively.
In a second approach fibrillization of Tau was monitored using transmission electron microscopy (TEM). Samples were placed onto carbon – coated grids and a negative staining was performed (1% Uac). The TEM analysis was done in cooperation with the Institute of Cell Biology, Histology & Embryology at the Medical University of Graz.
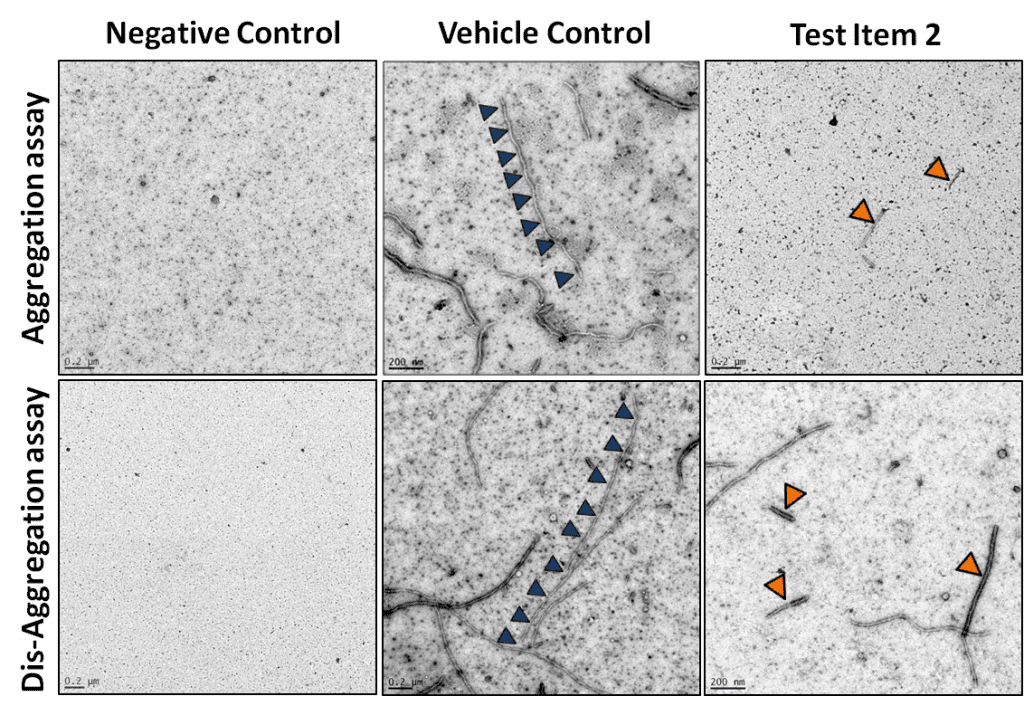
Figure 2: Test Item 2 reduced the size and number of Tau fibrils in the Tau aggregation (upper row) and dis-aggregation assay (lower row) in contrast to the Vehicle Control. Long Tau fibrils in the Vehicle Control are indicated by blue triangles and short ones after applying test Item 2 are indicated by orange triangles.
