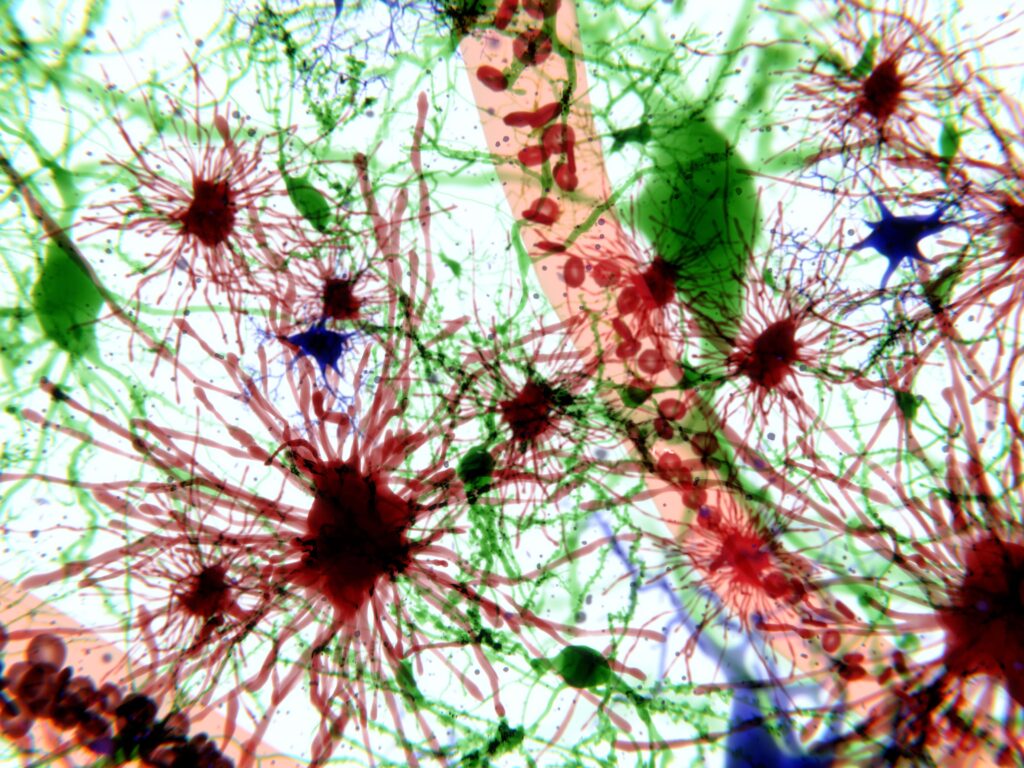
Researchers at Trinity Biomedical Sciences Institute (TBSI) have discovered a link between the presence of specialized immune cells, called microglia, and age-related neurological diseases. In their study, the scientists from TBSI and the University of Maryland School of Medicine observed that as the brain ages, cellular debris accumulates inside autofluorescent microglia, slowing their functioning and impeding their effectiveness in preventing neurological injuries and diseases. This new research, published in the journal Science Advances, highlights the need for further research into treatments targeting microglia in order to mitigate the effects of aging and disease on the brain.
Microglia and the Brain
Microglia are a unique, specialized type of immune cell found in the brain and spinal cord. They play crucial roles in supporting nerve cells, defending against invading microbes, clearing debris, and removing dying nerve cells through phagocytosis.
In healthy brain tissue, microglia help to maintain homeostasis by supporting the development and maintenance of synaptic connections within the brain. They also play a neuroprotective role: when the brain is faced with injury, infection, or disease, microglial cells release a range of molecules to promote or inhibit an inflammatory response.
Over time, however, and with advanced age, the accumulation of intrinsic and extrinsic stressors leads to higher levels of microglial dysfunction, negatively affecting neurological function. And new research indicates that age is not the only trigger for microglial dysfunction.
Role in Neurological Diseases
With age, microglia adopt a “unique, dysfunctional state” in brain cells. This state is directly linked to a number of problematic neurological impacts, including altered metabolic processes, increased cellular stress and damage, higher rates of fat and iron accumulation, and disproportionate immune responses.
One hallmark of this type of cellular dysfunction is the accumulation of lipofuscin, an autofluorescent lipopigment formed by lipids, metals, and misfolded proteins. In the past, scientists have observed that the accumulation of lipofuscin and autofluorescence in microglia increases as part of the physiological process of aging. But until recently, scientists had not been able to establish a functional relationship between lipofuscin granule accumulation and associated autofluorescence in microglia. These had previously been understood exclusively as secondary consequences of brain aging or neurodegenerative disease.
A new study published in Science Advances could change that.
Autofluorescence and Inflammation
The study’s lead author, David Loane, Assistant Professor of Neuroscience at Trinity’s School of Biochemistry and Immunology in TBSI, noted that he and his team were able to observe a distinct, pronounced increase in microglial autofluorescence (and associated inflammation) in genetic risk factor models of Alzheimer’s disease. The research team further demonstrated that environmental exposure to acute traumatic brain injury in animals also accelerates the age of onset and the tissue-wide distribution of autofluorescent microglia by increasing oxidative stress damage in injured animals’ brains.
Put another way, Loane’s research suggests that higher levels of microglial autofluorescence, triggered by lipofuscin accumulation, may play a much more active role in neurodegeneration than previously understood.
And Loane and his team didn’t just demonstrate a link between microglial autofluorescence and neurodegeneration. They also learned that drug-assisted microglial replacement can reverse the accumulation of autofluorescent microglia and, in doing so, can also reverse some of the neurological dysfunction this accumulation causes.
Future Implications
While more research is still needed to fully understand the role these cells play in aging and disease, Loane and his team’s work represents a significant step forward in our understanding of some of the underlying mechanisms of neurodegeneration. The results indicate that targeting damaging, inflammatory sub-populations of microglia could represent a promising new method for treating aging-related diseases.
Scantox is a part of Scantox, a GLP/GCP-compliant contract research organization (CRO) delivering the highest grade of Discovery, Regulatory Toxicology and CMC/Analytical services since 1977. Scantox focuses on preclinical studies related to central nervous system (CNS) diseases, rare diseases, and mental disorders. With highly predictive disease models available on site and unparalleled preclinical experience, Scantox can handle most CNS drug development needs for biopharmaceutical companies of all sizes. For more information about Scantox, visit www.scantox.com.