Tau proteins belong to the family of microtubule-associated proteins and play an important role in stabilizing the neuronal microtubules network. They are the major constituents of intraneuronal and glial fibrillar lesions described in Alzheimer’s disease and numerous neurodegenerative disorders referred to as ‘tauopathies’, including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Pick’s disease (PiD), argyrophilic grain disease, as well as the inherited frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17).
Molecular analysis revealed that hyperphosphorylation might be the important event leading to tau aggregation resulting in neurodegeneration and dementia. Development of new compounds capable of preventing tau hyperphosphorylation is an increasingly hot topic.
Thus, reliable models are needed that reflect tau hyperphosphorylation in human diseases. For this purpose, we generated the stably transfected SH-SY5Y cell line over-expressing the longest human Tau441 isoform comprising two disease related mutations (V337M/R406W). The phosphorylation pattern of tau in SH-SY5Y-TMHT441 cells can be reliably modulated by several kinase inhibitors. Effects on tau phosphorylation (e.g. at Thr181, Ser202, Thr231/Ser235, Ser262 and Ser396/Ser4049) can be determined by various methods including immunosorbent assay (MSD), immunoblot analysis or mass spectrometry (Löffler T. et al, J Mol Neurosci 2012 May;47(1):192-203).
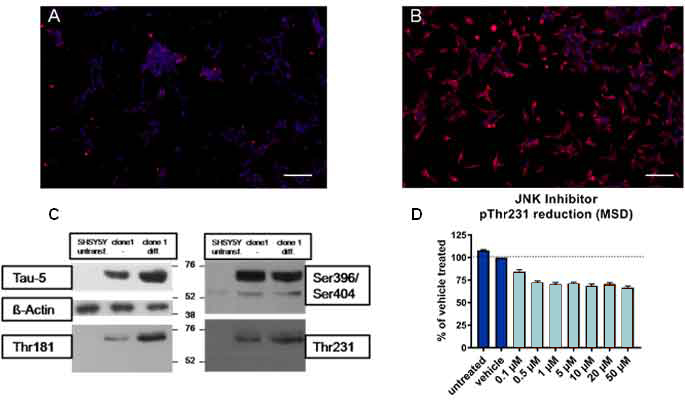
Figure: Tau hyperphosphorylation in SH-SY5Y-TMHT441 cells. Immunocytochemistry showing overexpression of human tau in (A) untransfected and (B) transfected SH-SY5Y cells (Scale bar 100 µm). Human tau is indicated in red, DAPI in blue. (C) Tau phosphorylation in control SH-SY5Y cells, in undifferentiated and differentiated SH-SY5Y-CMV-TMHT441 cells was examined by immuno blot analysis. (D) pThr231 was reduced in SH-SY5Y-CMV-TMHT441 cells after treatment with the JNK inhibitor SP600125 as assessed by an immunosorbent assay (MSD).
