CCl4-induced liver fibrosis
Liver fibrosis occurs in most types of chronic liver diseases like Hepatitis B and C, viral infections by cytomegalovirus or Epstein Barr virus, alcoholic liver disease, non-alcoholic fatty liver disease, haemochromatosis, Wilson’s disease and many more. In humans, researchers were already able to show that liver fibrosis is reversible, fostering the development of anti-fibrotic drugs. To test the efficacy of these new drugs, proper preclinical models are needed. To model liver fibrosis in vivo, mice can be systemically injected with carbon tetrachloride (CCl4), an organochloride known as one of the most potent hepatotoxins which induces liver fibrosis.
The most important characteristics of CCl4-induced mice are:
- Altered liver to body weight ratio
- Increased expression of liver disease markers
C57Bl/6 mice at an age of 7 weeks were intraperitoneally treated three times per week with CCl4 or vehicle for a total of 9 weeks to induce liver fibrosis. In addition to the liver to body weight ratio, disease markers can be analyzed in the liver, including, but not limited to Col1a1, Acta2, hydroxyproline and smooth muscle actin (SMA). Markers can be evaluated on mRNA or protein level by real time RT-PCR or protein assays as well as qualitative and quantitative immunofluorescent labeling. All markers are shown to be increased after CCl4 treatment (Figure 1).
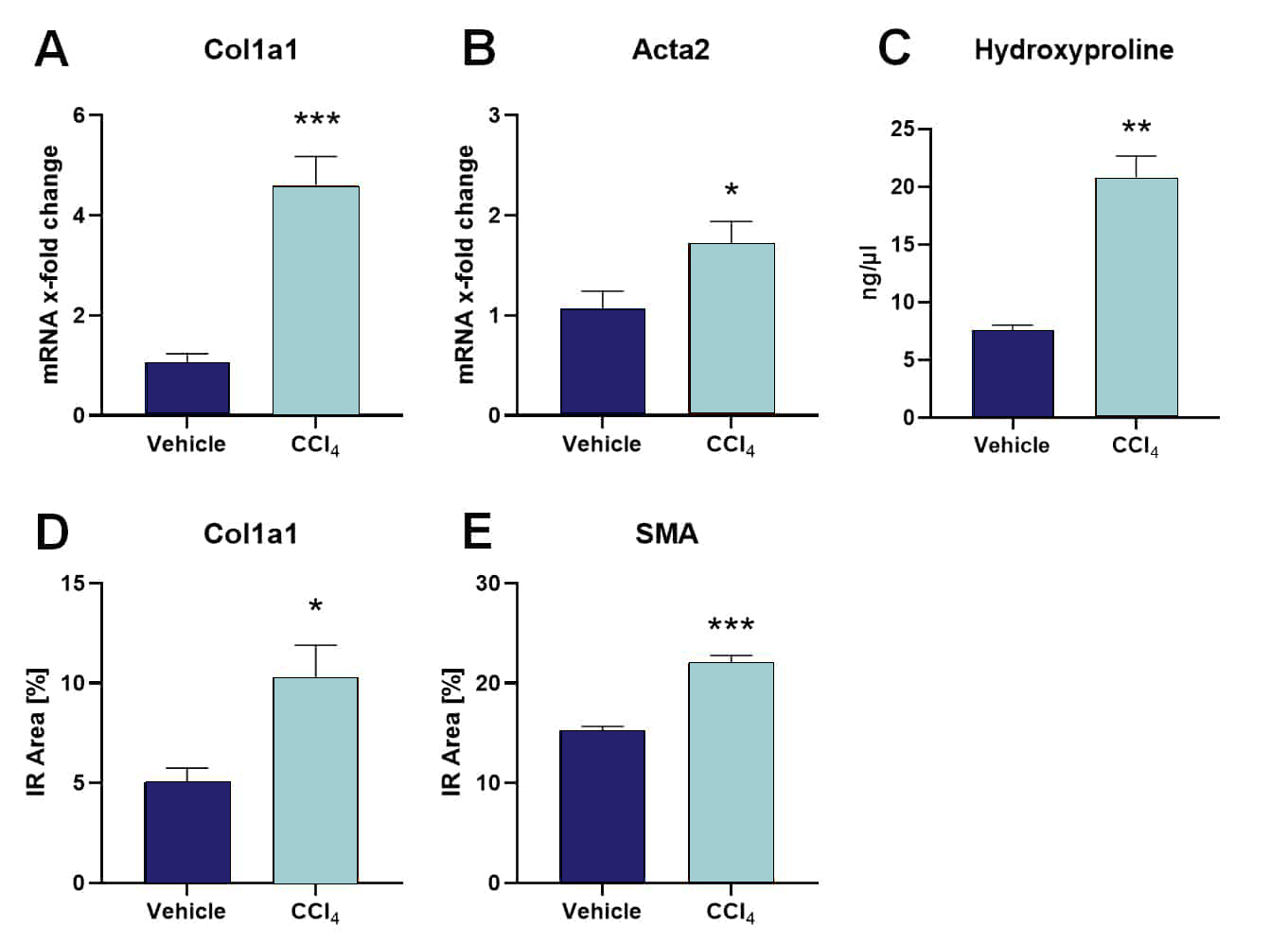
Figure: Expression levels of typical liver disease markers after CCl4 treatment. mRNA expression levels of Col1a1 (A) and Acta2 (B). Levels are presented as x-fold change using the 2^(-ΔΔCT) method in relation to the housekeeping gene HPRT and vehicle. C: Hydroxyproline protein levels in ng/µl. Quantification of Col1a1 (D) and SMA (E, smooth muscle actin) immunoreactive (IR) area in percent. A-E: All markers were measured in the liver after 9 weeks of CCl4 or vehicle treatment. Unpaired t-test; n = 5/6 per group; mean + SEM; *p<0.05, **p<0.01, ***p<0.001.
Scantox offers a custom-tailored study design for the induced liver fibrosis model, and we are flexible to accommodate your special interest. We are also happy to advise you and propose study designs. CCl4 induced mice are a valuable model to ensure a fast processing time for your study. Vehicle-injected wild type mice can serve as control needed for proper study design. The CCl4 model is therefore an excellent tool to evaluate the efficacy of new liver fibrosis treatments and to observe the time dependent effects of such a treatment.
We are happy to evaluate the efficacy of your compound in the CCl4-induced liver fibrosis animal model! The most common readouts include:
