AAV2 hA53T-α-syn Induced Mouse Model
Wild type C57BL/6 mice that receive a single, unilateral injection of AAV2 hA53T-α-syn (human α-synuclein with A53T mutation) into the substantia nigra selectively express hA53T-α-syn in the ipsilateral hemisphere.
The most important characteristics of AAV2 hA53T-α-syn-induced mice are:
- Expression of human A53T- α-synuclein in distinct brain regions
- Microgliosis
- Reduced tyrosine hydroxylase levels
Histological analysis of AAV2 hA53T-α-syn injected brains shows increased hA53T-α-syn protein levels in the substantia nigra as well as in the caudate putamen of the injected brain hemisphere.
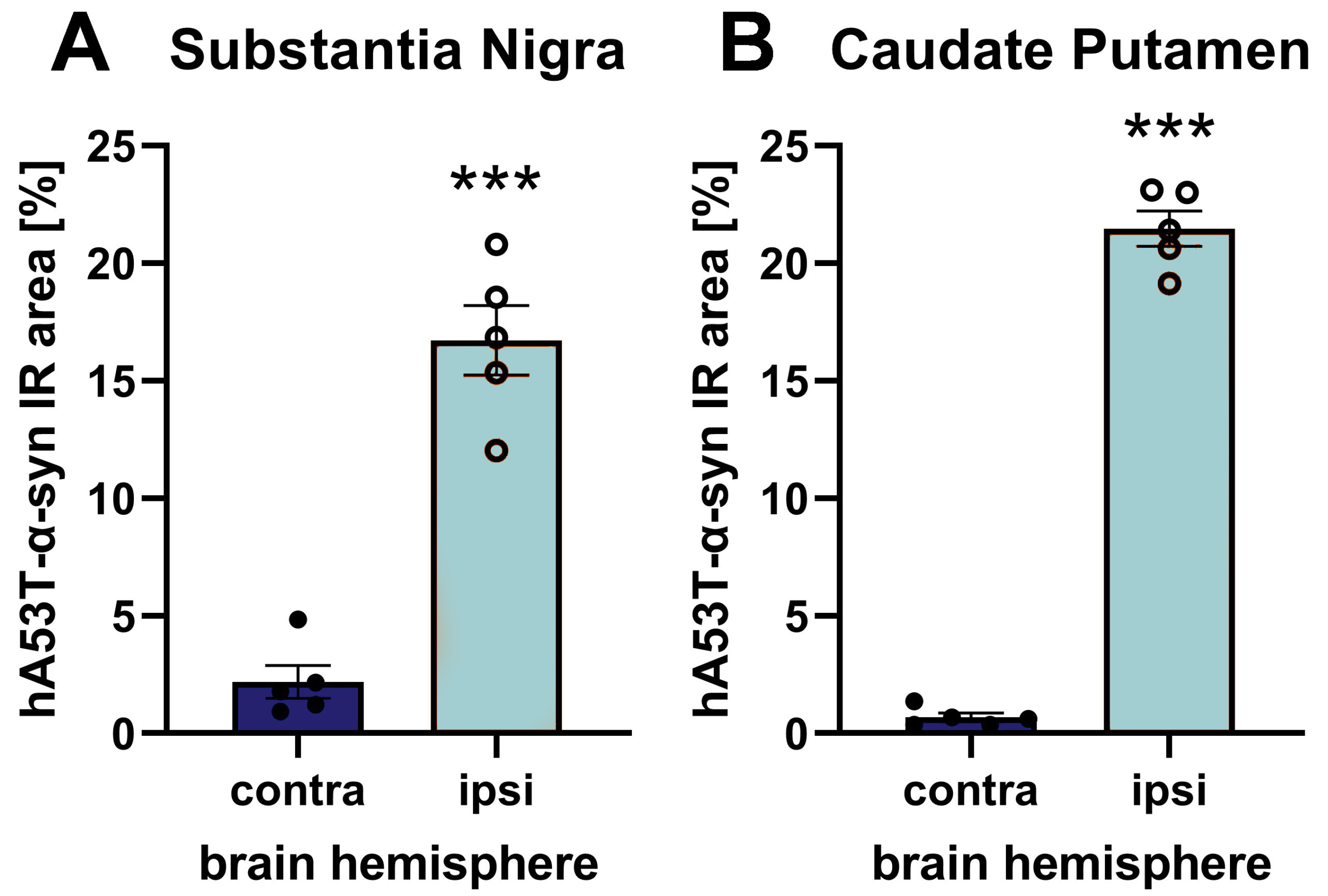
Figure 1: hA53T-α-syn immunoreactive area (IR) in the substantia nigra (A) and caudate putamen (B) of the contra- and ipsilateral hemisphere after unilateral AAV2 hA53T-α-syn injection into the substantia nigra of the ipsilateral hemisphere. Animals were euthanized 9 weeks after injection and brains were evaluated using a human-specific α-syn antibody. n = 5 / group; unpaired t-test; Mean ± SEM. ***p<0.001.
hA53T-α-syn expression further leads to increased activated microglia as marker of neuroinflammation and even strongly decreased tyrosine hydroxylase (TH) levels in the injected substantia nigra as evaluated by quantification of immunofluorescent labeling.
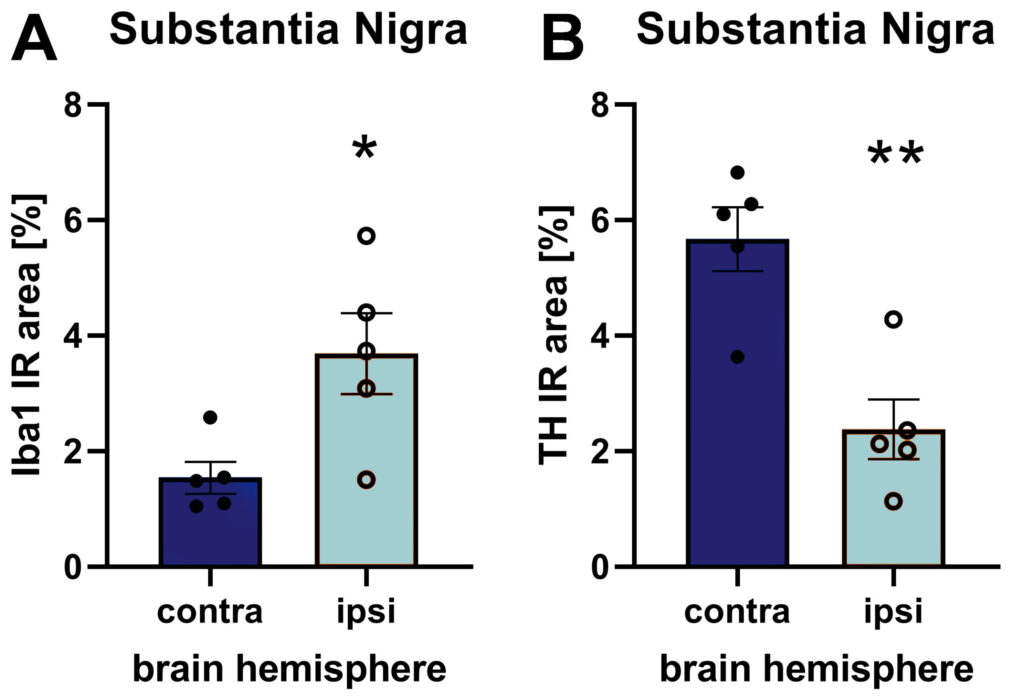
Figure 2: Iba1 (A) and TH (B) immunoreactive area (IR) in the substantia nigra of the contra- and ipsilateral hemisphere after unilateral AAV2 hA53T-α-syn injection into the substantia nigra of the ipsilateral hemisphere. Animals were euthanized 9 weeks after injection. n = 5 / group; unpaired t-test; Mean ± SEM. *p<0.05; **p<0.01.
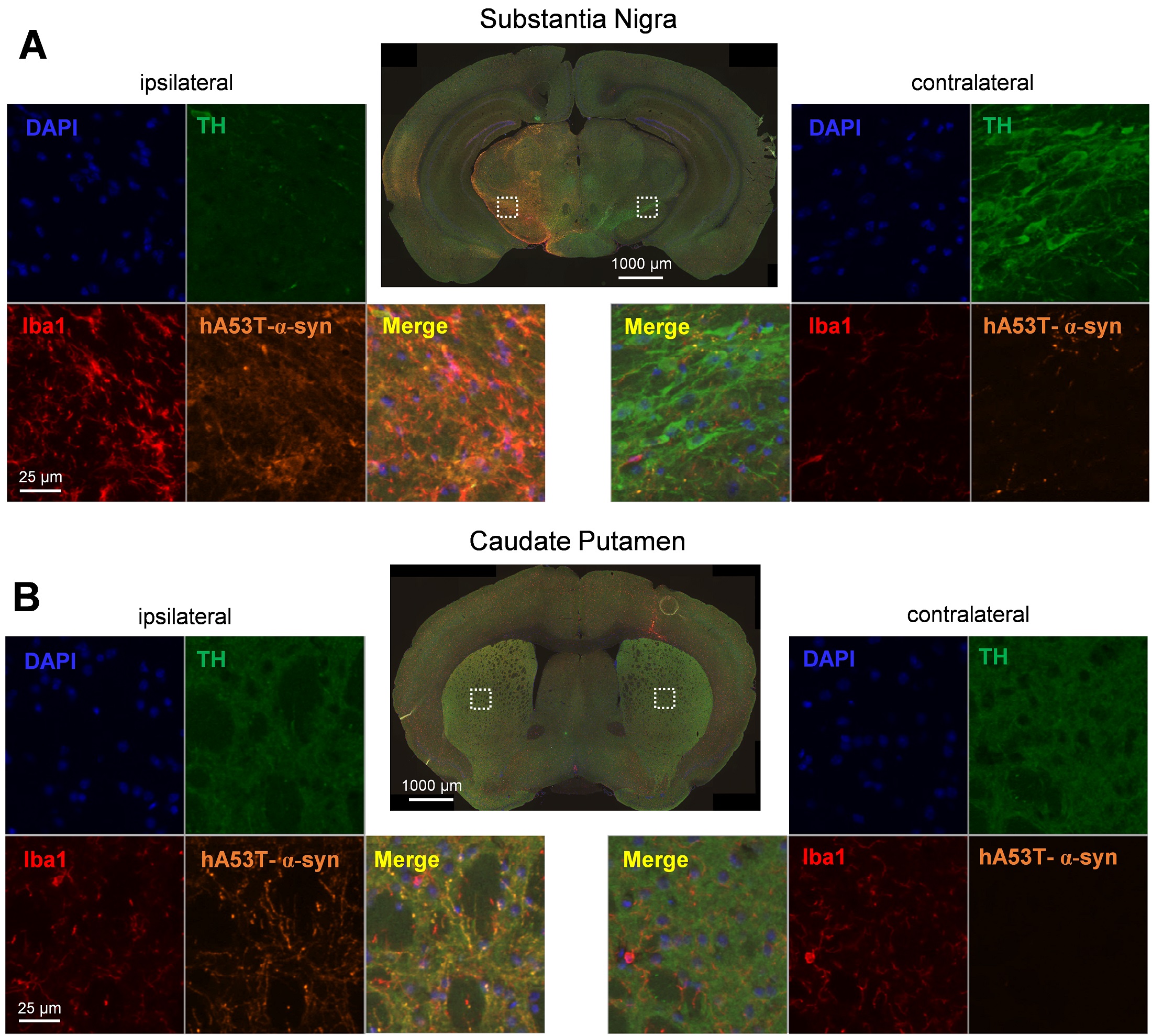
Figure 3: Representative images of hA53T-α-syn, tyrosine hydroxylase (TH), Iba1 and DAPI labeling in the ipsi- (left) and contralateral (right) hemisphere of the substantia nigra (A) and caudate putamen (B) after unilateral AAV2 hA53T-α-syn injection into the substantia nigra of the ipsilateral hemisphere.
MPTP-induced Mouse Model
Mice that receive acute, chronic or subchronic administration of the pyridine toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) selectively lose significant numbers of dopaminergic neurons in two midbrain structures, the substantia nigra (SN) and the ventral tegmental area (VTA). Loss of dopamine cells in the SN mimics the clinical condition of Parkinson’s disease and leads to motor dysfunction.
The dopaminergic loss in mouse VTA is of unknown relevance to Parkinson’s disease but may contribute to the cognitive deficits of Parkinson’s disease because of these neurons’ projections to the frontal cortex.
The most important characteristics of MPTP-lesioned mice are:
- Reduced activity
- Loss of dopaminergic neurons in substantia nigra and VTA
- Reduction of DOPAC and HVA in the caudate putamen
- Reduction of TH in the substantia nigra
Behavioral analysis of MPTP-lesioned mice two days after treatment results in a significantly reduced activity compared to sham-lesioned animals (Fig.1)
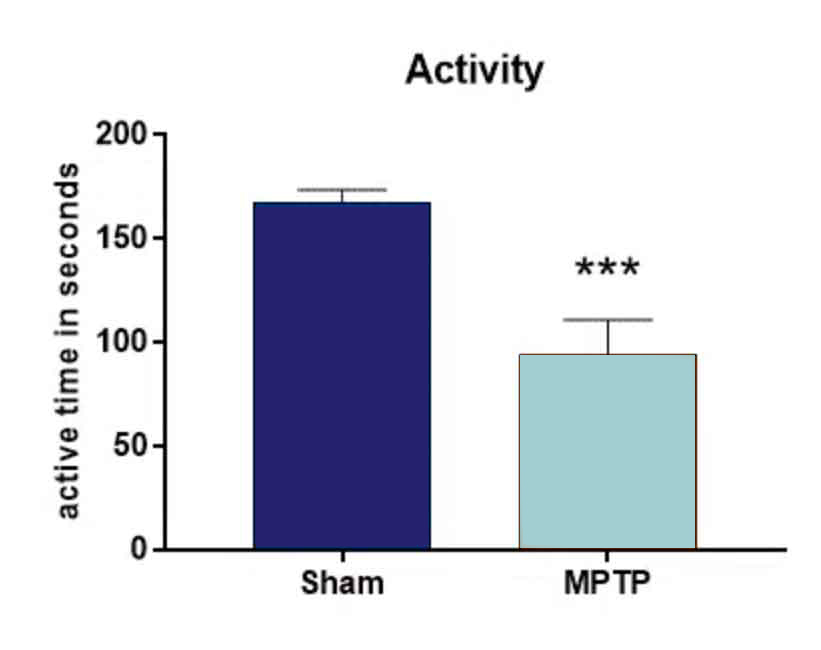
Figure 1: Open Field test. Animals were injected with 4 x 20 mg/kg MPTP or vehicle at one day. Two days after treatment, animals were tested in the Open field test for activity. n = 9-10 per group; unpaired t-test; Mean + SEM. ***p<0.001.
Quantification of tyrosine hydroxylase (TH) in the substantia nigra after MPTP-lesioning results in significantly reduced TH levels compared to sham-lesion control mice (Fig.2).
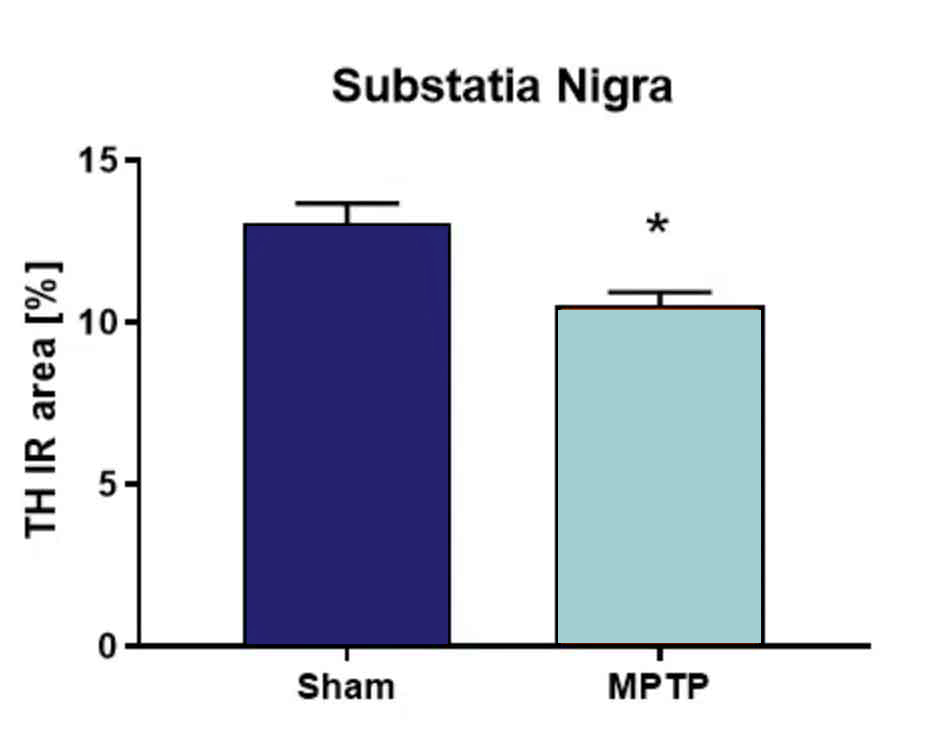
Figure 2. Tyrosine hydroxylase (TH) quantification in the substantia nigra after MPTP lesion. Animals were injected with 4 x 20 mg/kg MPTP or vehicle at one day. Six days after treatment, animals were sacrificed and the substantia nigra analyzed for TH levels. n = 3-5 per group; unpaired t-test; Mean + SEM. *p<0.05.
MPTP-treated mice are therefore a suitable model to study motor deficits and the loss of dopaminergic neurons as well as possible influences of drugs on these parameters.
6-OHDA Lesion Model
When unilaterally injected into specific brain regions of the rodent brain, 6-OHDA induces damage to catecholaminergic neurons by oxidative stress. Depending on dose and injection site, this leads to a severe loss of dopaminergic neurons, often demonstrated by the loss of tyrosine hydroxylase (TH) immunoreactivity. This pathology mimics one major hallmark of PD, dopamine deficiency in the mesostriatal system. The 6-OHDA lesion has therefore been used as a model of PD for more than 35 years.
Popular injection sites for 6-OHDA are the medial forebrain bundle, different parts of the striatum (single or multiple injection sites), or the substantia nigra.
The most important characteristics of 6-OHDA-lesioned rats when injected into the MFB are:
- Lateral behavioral deficits in the cylinder and rotation test
- Reduced tyrosine hydroxylase levels in caudate putamen
- Reduced DAT levels in caudate putamen
- Astrocytosis and activated microglia in caudate putamen
Unilateral 6-OHDA lesioning of the medial forebrain bundle (MFB) results in strong lateral behavioral deficits 3 weeks after placing the lesion as analyzed by cylinder and rotation test (Fig. 1).
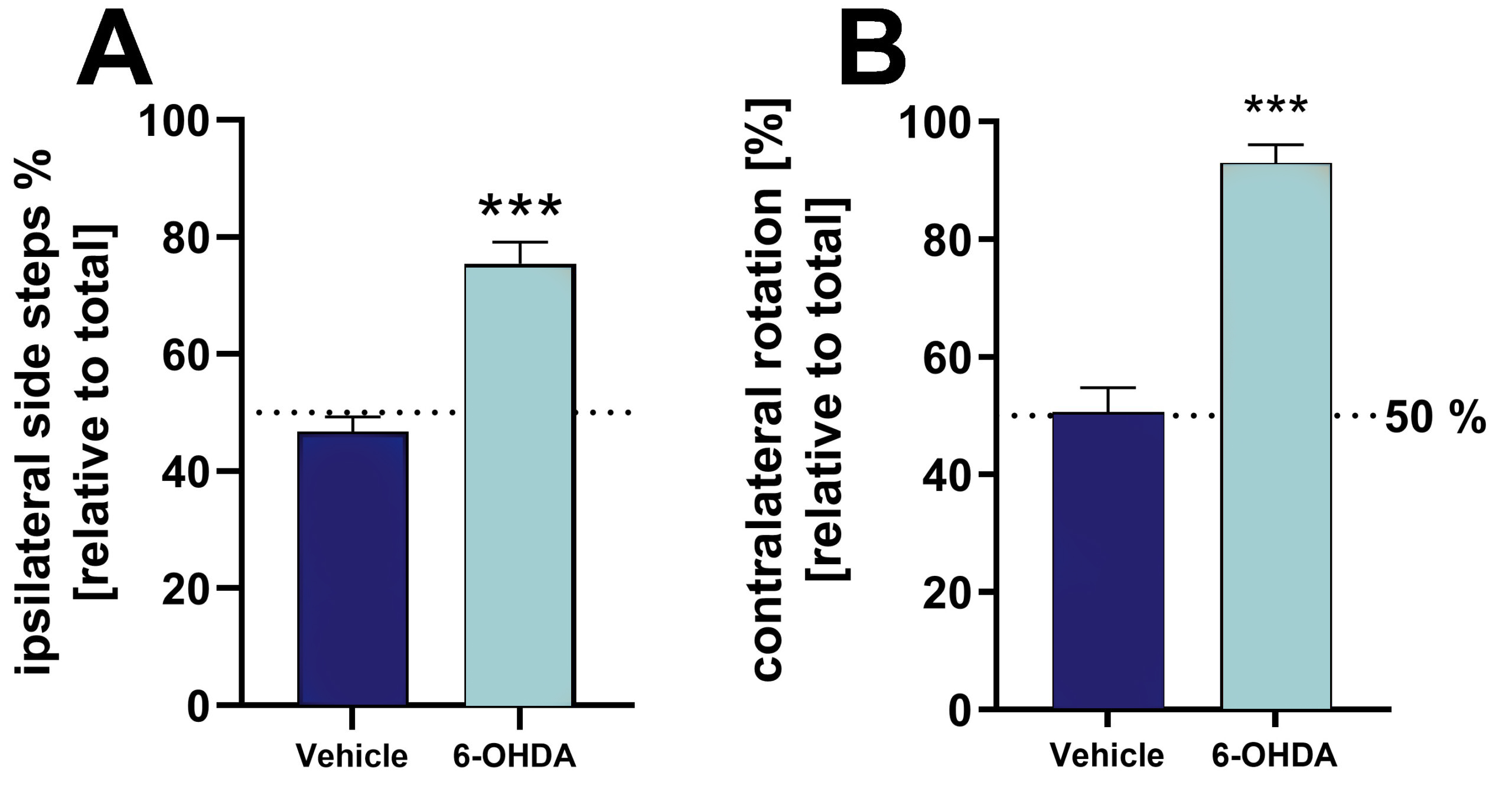
Figure 1: Validation of 6-OHDA lesion by cylinder and rotation test of 6-OHDA-injected and vehicle-injected rats. A: Ipsilateral side steps [%] relative to total side steps in the cylinder test. B: Relative contralateral rotations in the rotation test. Tests were performed 3 weeks after 6-OHDA lesioning. For the rotation test, animals were treated with apomorphine to induce rotation behavior. Mean + SEM. Unpaired t-test. n = 8 (vehicle) and 19 (6-OHDA); ***p<0.001.
L-DOPA/benserazide treatment at 3 concentrations can reverse the lateral behavioral effects observed in the cylinder test (Fig. 2A). Further evaluation of 6-OHDA rats in the rotation test shows that the relative number of contralateral rotations is unaffected by different L-DOPA concentrations (Fig. 2B).
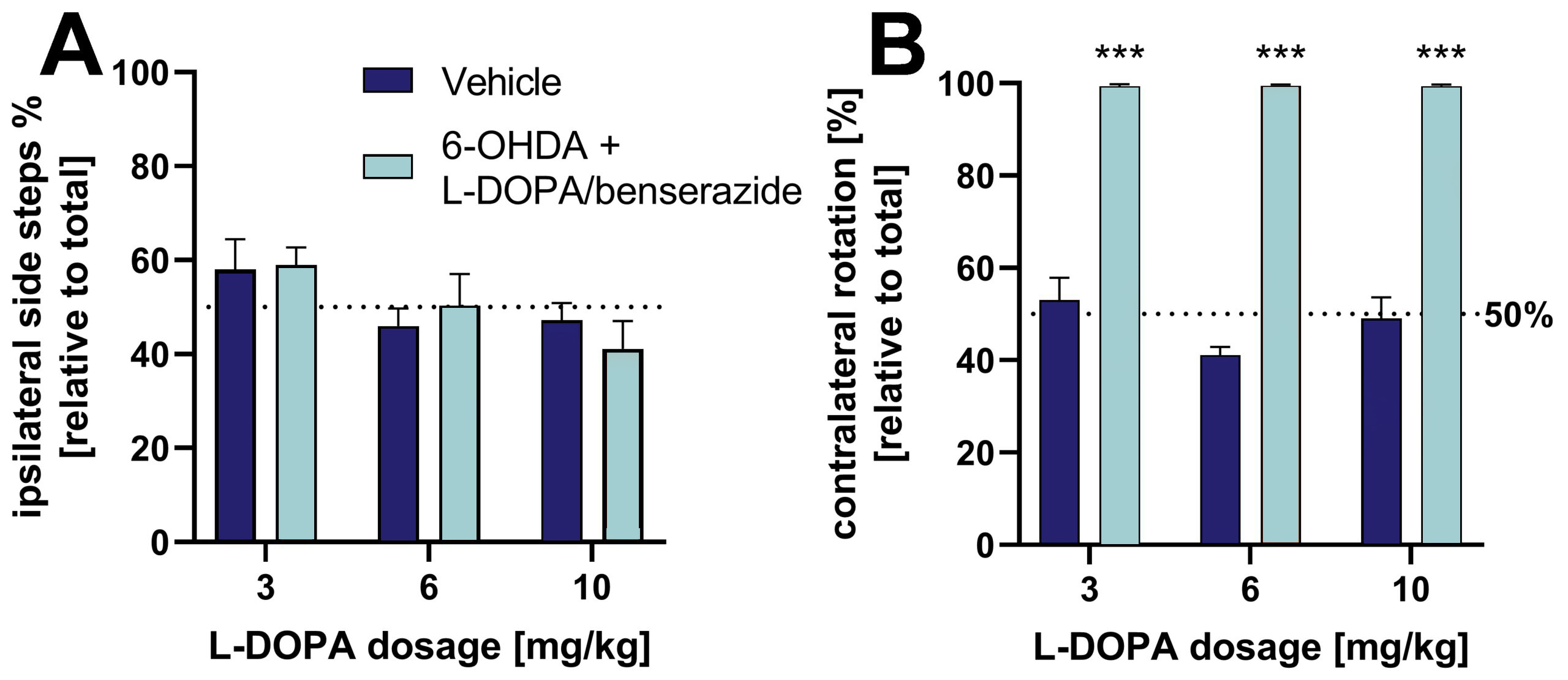
Figure 2: Effect of L-DOPA/benserazide on lateral behavior of 6-OHDA-lesioned rats in the cylinder and rotation test. A: Ipsilateral side steps [%] relative to total side steps in the cylinder test. B: Relative contralateral rotations in the rotation test. For the rotation test, animals were treated with apomorphine to induce rotation behavior. Mean + SEM. Two way ANOVA with Tukey’s and Sidak’s post hoc test. n = 8 (all vehicle groups) and 16 (all 6-OHDA groups); ***p<0.001.
Histological evaluation of the tyrosine hydroxylase (TH)- and DAT-positive immunoreactive area (IR) in the caudate putamen 5 weeks after 6-OHDA lesioning of the MFB reveals strongly reduced TH and DATA levels in the lesioned side of the brain (Fig. 3A, B). Histological evaluation of the glial fibrillary acidic protein (GFAP)-, and Iba-1 positive immunoreactive area in the caudate putamen after 6-OHDA lesioning of the MFB reveals increased GFAP and Iba-1 levels in the caudate putamen of the lesioned side of the brain (Fig. 3C, D). These data demonstrate that 6-ODHA lesioning induces neuroinflammation as shown by astrocytosis and activated microglia.
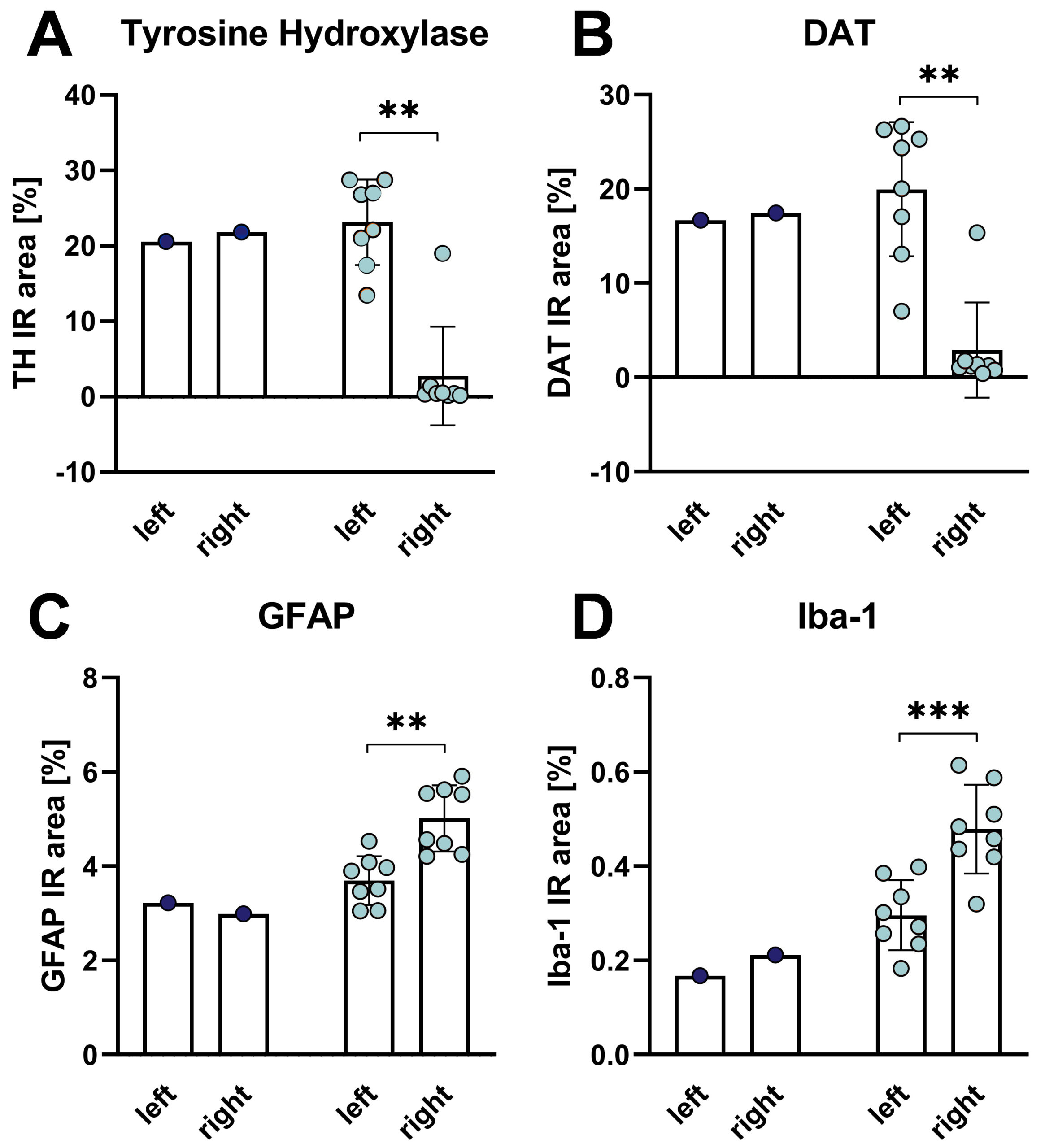
Figure 3: Histological evaluation of the caudate putamen for tyrosine hydroxylase (TH, A), DAT (B), GFAP (C) and Iba-1 (D) IR area after 6-OHDA lesion of the MFB in rats. Unpaired t-test with 6-OHDA groups only. 6-OHDA: n = 8; vehicle: n = 1. Mean + SEM. **p<0.01, ***p<0.001. left: untreated side; right: treated side. Blue: vehicle group, orange: 6-OHDA group.
Haloperidol Induced Catalepsy
Catalepsy is a neuronal condition that can be observed in Parkinson’s disease, epilepsy, catatonia but also as adverse reaction to prescribed medications such as medications against schizophrenia. Catalepsy is characterized by seizures with a loss of sensation and consciousness accompanied by rigidity of the body. By treating rats with the dopamine D2 receptor antagonist haloperidol catalepsy can be acutely induced for several hours, mimicking the typical symptoms.
Sprague Dawley rats are subcutaneously injected with 1 mg/kg haloperidol. After 30 minutes, catalepsy is measured and additionally, animals showing the full spectrum of catalepsy are treated orally with the positive compound or vehicle. Animals can be retested for catalepsy at several time points after drug administration.
The most important characteristics of haloperidol-treated rats are:
- Loss of consciousness
- Body rigidity
Catalepsy can be evaluated by gently placing the front limbs of a rat over an 8 cm high horizontal bar; catalepsy can be measured as time animals spent motionless. A cut-off time of 120 seconds is used.
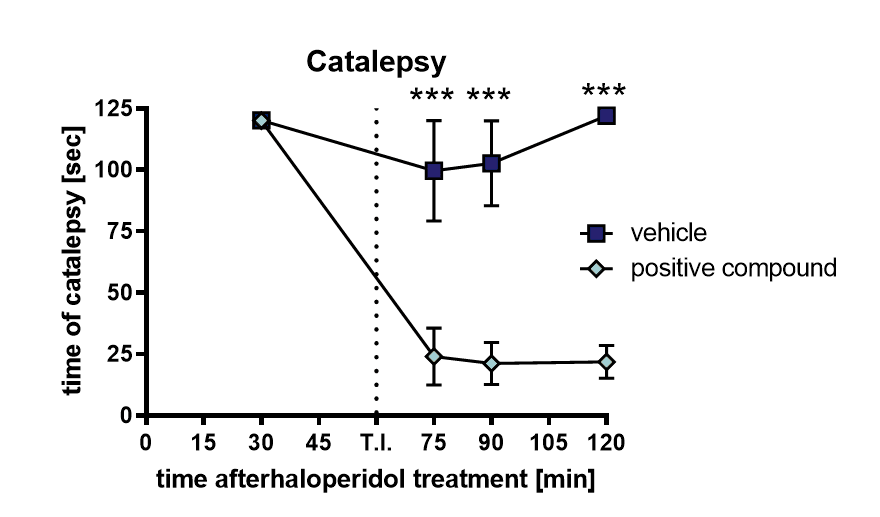
Figure 1: Haloperidol-induced catalepsy after treatment with a positive compound: Time animals spent rigid after subcutaneous treatment with 1 mg/kg haloperidol, followed by oral treatment with a positive compound. Animals were repeatedly tested for catalepsy. n = 10. Mean ± SEM. Two-way ANOVA with Bonferroni’s repeated measure post hoc test. ***p<0.001. T.I.: test item / positive compound.
Scantox offers a custom-tailored study design for all induced PD model, and we are flexible to accommodate to your special interest. We are also happy to advise you and propose study designs. All induced models show a relevant Parkinson’s disease (PD) phenotype shortly after treatment. This grants a remarkable fast processing time of your PD study. Furthermore, vehicle-injected wild type mice and/or the contralateral hemisphere of the injected animals can serve as control needed for proper study design.
We are happy to evaluate the efficacy of your compound in an induced PD animal model! The most common readouts depend on the model and include:
